Spinning Disk- Olympus (Irchel)
The Olympus IXplore SpinSR10 super resolution imaging system is a high-end real time spinning disk confocal imaging system.
It is equipped with two sCMOS cameras, which allow fast and sensitive simultaneous acquisition of two-color labelled (live) samples.
Designed for experiments involving live cells, the spinning disk (YOKOGAWA CSU-W1) reduces phototoxicity and bleaching while the Olympus Z-drift compensator (IX3-ZDC2) maintains focus.
The photomanipulation unit (Rapp OptoElectronic) allows localized and controlled photostimulation as well as damage/ablation in biological samples.
The stage top incubation cellVivo system allows control of temperature and CO2 levels enabling live cell experiments.
Location
University Zurich, Irchel Campus, Room Y42-H-83.
Training Request
Follow this link to apply for an introduction to the microscope.
Technical Specifications
Microscope
Light Sources and Lasers
- Laser lines: 405 nm (50 mW), 488 (100mW), 561 nm (100 mW), 640nm (100mW), 445nm (75nm) and 514nm (40mW).
- Photomanipulation unit: 355 nm (pulsed, UGA‑42 Caliburn), 405 nm (100 mW), 473 nm (100mW)
- CoolLED’s pE-300 LED illumination for widefield fluorescence microscopy (3 LEDs). Spectral coverage from the UV (DAPI excitation) to the red region (Cy5 excitation)
Objectives
| Name | Magnification | NA | Immersion | WD (mm) |
|---|---|---|---|---|
| UPLSAPO UPlan | 4x | 0.16 | Air | 13 |
| UPLXAPO20X | 20x | 0.8 | Air | 0.6 |
| UPLAN S Apo | 40x | 0.95 | Air | 0.18 |
|
Silicon oil Immersion |
||||
| UPLSAPO UPlan S Apo | 30x | 1.05 | Silicon oil | 0.8 |
|
UPLSAPO UPlan S Apo |
60x | 1.3 |
Silicon oil |
0.3 |
|
UPLSAPO UPlan S Apo |
100x | 1.3 |
Silicon oil |
0.2 |
Additional objectives (not currently installed)
| Name | Magnification | NA | Immersion | WD (mm) |
|---|---|---|---|---|
| LUCPLFN PH | 20x | 0.45 | Air |
6.60 - 7.80 |
Fluorescence Filters
Confocal Imaging Filters (implemented in CSU-W1)
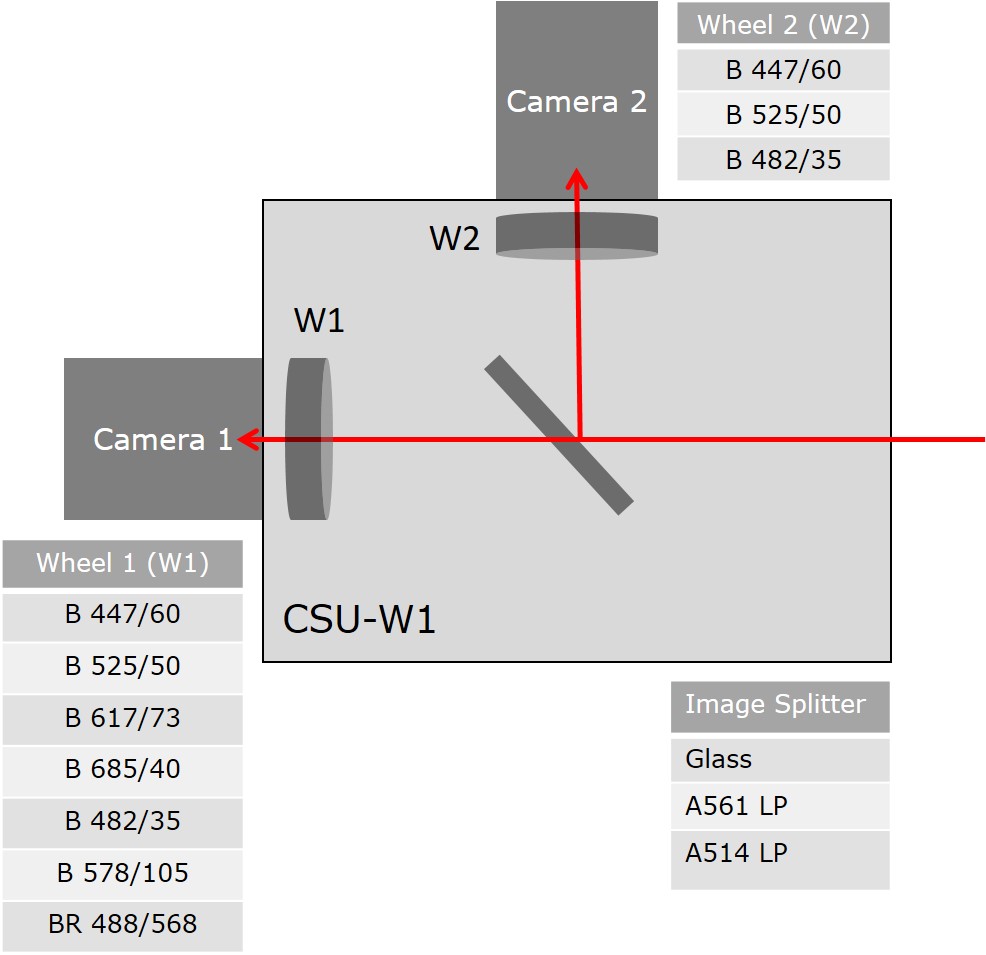
Recommended Imaging Settings
| Name/ Excitation Lasers |
Image splitting dichroic
|
Camera / Wheel 1 | Camera / Wheel 2 |
|---|---|---|---|
| 405/561 | A561 LP | BP 617/73 | BP 447/60 |
| 488/640 | A561 LP | BP 685/40 | BP 525/50 |
| 405/488 | A514 LP | BP 525/50 | BP 447/60 |
| 405 | Glass | BP 447/60 | - |
| 488 | Glass | BP 525/50 | - |
| 561 | Glass | BP 617/73 | - |
| 640 | Glass | BP 685/40 | - |
| 445 | Glass | BP 482/35 | - |
| 514 | Glass | BP 578/105 | - |
Widefield
For further details please contact the responsible persons.
Camera Systems
- 2x Hamamatsu ORCA-Fusion sCMOS
- 2304 x 2304 pixel (6.5 x 6.5um pixel size)
- up to 89.1 fps @ 16-bit (at 2304 x 2304 pixel)
Accessories
- Inserts for ibidi slides, well plates and 35mm dishes
- Incubation system for live cell imaging (cellVIVO)
- Allows for a variety of experimental protocols with 0 - 20 % CO2.
- Reliable control of temperature and CO2 with up to 0.05 °C accuracy
Literature and Links
Responsible Persons
If you have questions about the device please contact the responsible person.
Example of Materials and Methods when using this instrument (please adjust accordingly with your specific settings)
Single plane / Maximum intensity projections (MIP) of x um stacks were acquired using Olympus IXplore SpinSR10 spinning disk confocal microscope, equipped with a Yokogawa CSU-W1 50 μm pinhole disk and a 00x magnification x air / immersion media objective. Sequential laser excitation was performed using 405nm, 488nm, 561nm. Emission was detected through the following band pass filters Fluorophore 1 (447/60), Fluorophore 2 (525/50) Fluorophore 3 (617/73) with a Hamamatsu ORCA-Fusion sCMOS camera (2304 x 2304 pixel, 6.5 x 6.5um pixel size).
Make sure you acknowledge the Center for Microscopy and Image Analysis in your publications (papers, posters) as well as internal lab/institute communications (comitte meetings, lab meetings, etc) to support us!
How to acknowledge contributions of the Center for Microscopy